103855
论文已发表
提 交 论 文
注册即可获取Ebpay生命的最新动态
注 册
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

使用加载中药紫草素 (Shikonin) 的热敏胶束成功施行针对乳腺癌的体内超热疗
Authors Su Y, Huang N, Chen D, Zhang L, Dong X, Sun Y, Zhu X, Zhang F, Gao J, Wang Y, Fan K, Lo P, Li W, Ling CQ
Received 18 January 2017
Accepted for publication 3 April 2017
Published 29 May 2017 Volume 2017:12 Pages 4019—4035
DOI http://doi.org/10.2147/IJN.S132639
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Bhavesh Kevadiya
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Abstract: The Chinese traditional medicine Shikonin is an ideal drug
due to its multiple targets to tumor cells. But in clinics, improving its
aqueous solubility and tumor accumulation is still a challenge. Herein, a
copolymer with tunable poly(N-isopropylacrymaide) and polylactic acid block
lengths is designed, synthesized, and characterized in nuclear magnetic
resonance. The corresponding thermosensitive nanomicelle (TN) with well-defined
core-shell structure is then assembled in an aqueous solution. For promoting
the therapeutic index, the physical-chemistry properties of TNs including
narrow size, low critical micellar concentration, high serum stability, tunable
volume phase transition temperature (VPTT), high drug-loading capacity, and
temperature-controlled drug release are systematically investigated and
regulated through the fine self-assembly. The shikonin is then entrapped in a
degradable inner core resulting in a shikonin-loaded thermosensitive
nanomicelle (STN) with a VPTT of ~40°C. Compared with small-molecular shikonin,
the in vitro cellular internalization and cytotoxicity of STN against breast
cancer cells (Michigan Cancer Foundation-7) are obviously enhanced. In
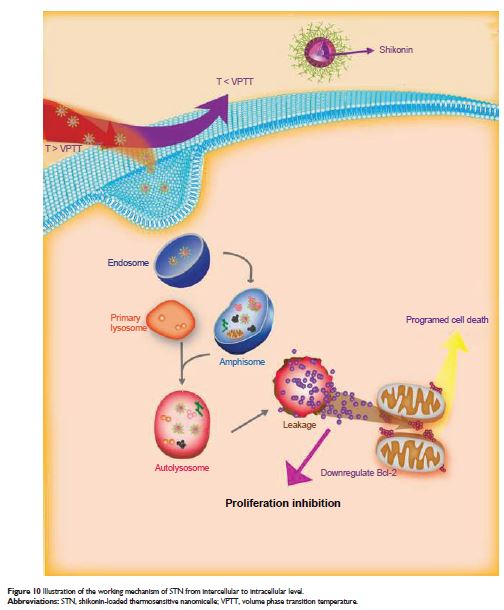
addition, the therapeutic effect is further enhanced by the programmed cell
death (PCD) specifically evoked by shikonin. Interestingly, both the
proliferation inhibition and PCD are synergistically promoted as T > VPTT,
namely the temperature-regulated passive targeting. Consequently, as
intravenous injection is administered to the BALB/c nude mice bearing breast
cancer, the intra-tumor accumulation of STNs is significantly increased as T
> VPTT, which is regulated by the in-house developed heating device. The in
vivo antitumor assays against breast cancer further confirm the synergistically
enhanced therapeutic efficiency. The findings of this study indicate that STN
is a potential effective nanoformulation in clinical cancer therapy.
Keywords: thermosensitive micelle, shikonin,
breast cancer, intra-tumor accumulation, critical micelle concentration,
hyperthermal therapy, in vivo